Publications
† Denotes equal authorship. * Denotes corresponding authorship.
You can also check my Google Scholar and ORCID profiles.
[12] Submitted, Chemical Science.
[11] Barth, A. T.* “Shining light on waste: Photochemical strategies to reduce and transform plastic pollution.” RSC Sustainability, 2025, 3, 4866-4869.
Photochemical strategies offer immense promise in degrading plastic waste into oxidized small molecules. I review efforts to valorize these materials using light-activated reactions, highlighting the global implications of this approach.
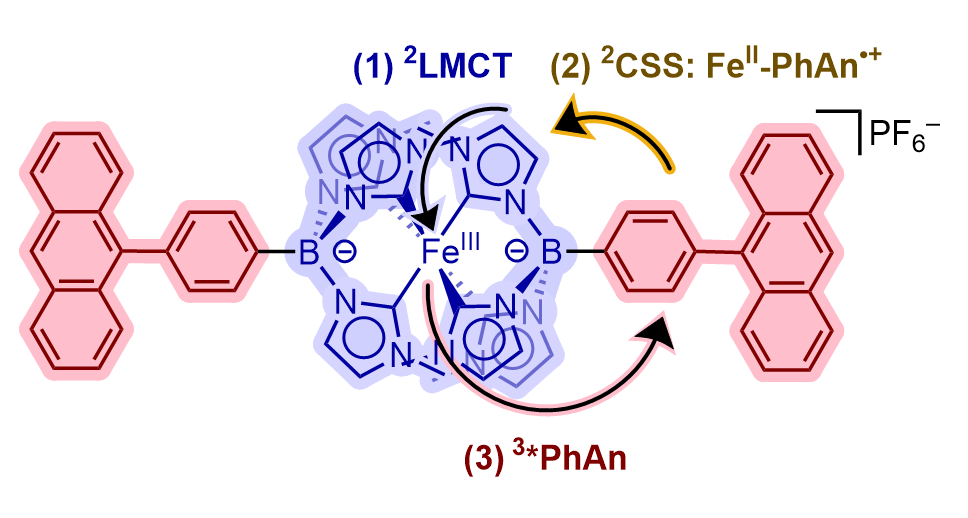
[10] Barth, A. T.*. and Castellano, F. N. “Chasing waterfalls: A cascade mechanism to generate triplets from ²LMCT states” ACS Cent. Sci. 2025, 11, 10, 1802–1804.
Ligand-to-metal charge transfer (LMCT) excited states represent a new route to access emissive states in earth-abundant complexes, but they can be prohibitively short-lived. These donor-acceptor dyads offer an electron-transfer strategy to generate persistent (μs) anthracene-localized triplet states.
[9] Arteta, S.; Deegbey, M; Durand, N.; Kibbe, R; Floß, J.; Barth, A. T.; Jakubikova, E; Reiser, O; Muddiman, D.C.; Castellano, F.N.* “Mechanistic insights to visible-light induced ATRA reaction powered by the symbiotic relationship between Cu(II)/Cu(I) phenanthroline-based complexes” (Article ASAP, ACS Catalysis)
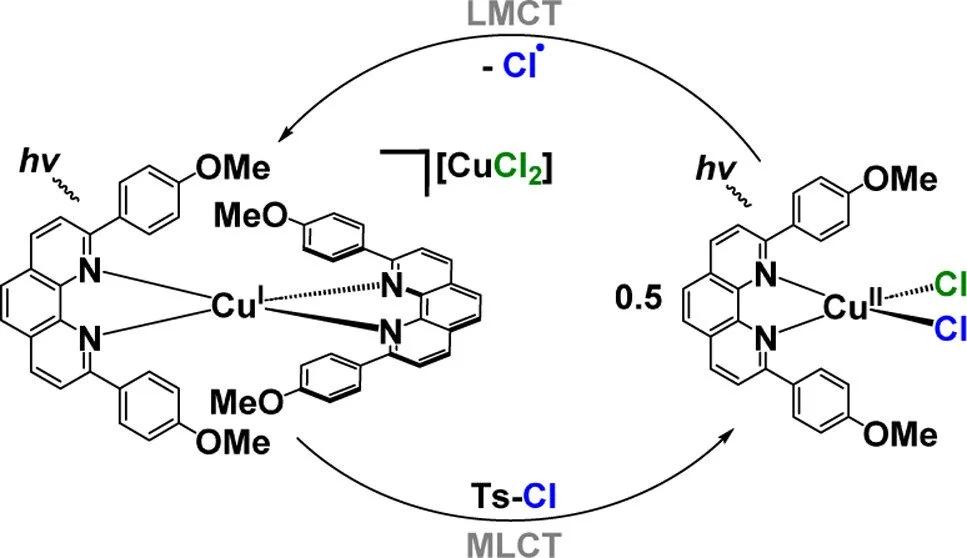
Cu(I) platforms are ubiquitous in LMCT photocatalysis, but spectroscopic monitoring of their role in photochemical reactions is obscured by the paramagnetic influence of Cu(II). We establish a symbiotic photocycle that assigns the role of both oxidation states in Atom Transfer Radical Addition (ATRA) reactions.
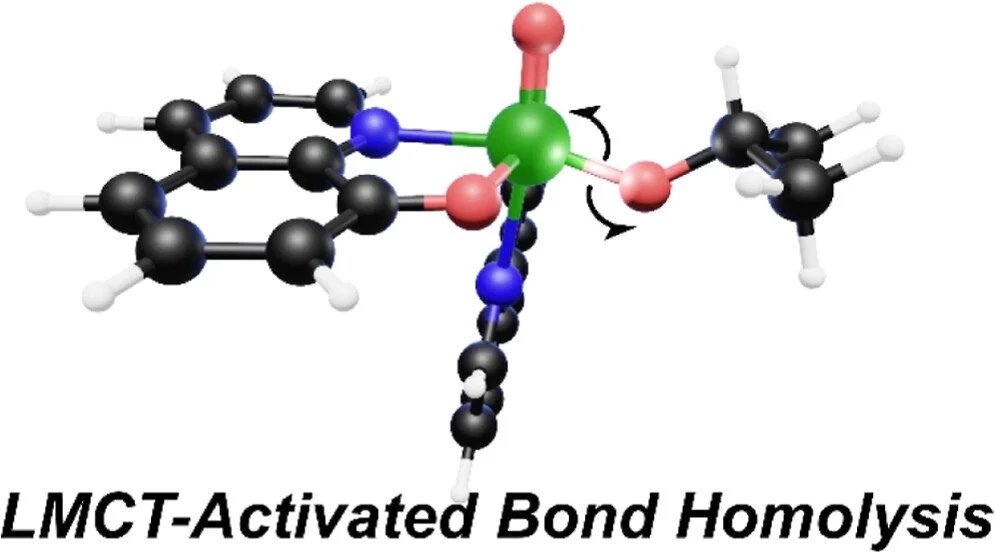
[8] A. T. Barth†, A. J. Pyrch†, C. T. McCormick, E. O. Danilov, F. N. Castellano* “Excited State Bond Homolysis of Vanadium(V) Photocatalysts for Alkoxy Radical Generation”. J. Phys. Chem. A 2024, 128, 36, 7609–7619.
Vanadium(V) has recently attracted interest as a base-metal platform for valorizing nonbiodegradable plastics. We probe intramolecular homolysis as an activation strategy for this desired reactivity and identify wavelength-dependent features in the excited-state profile using ultrafast TA spectroscopy.
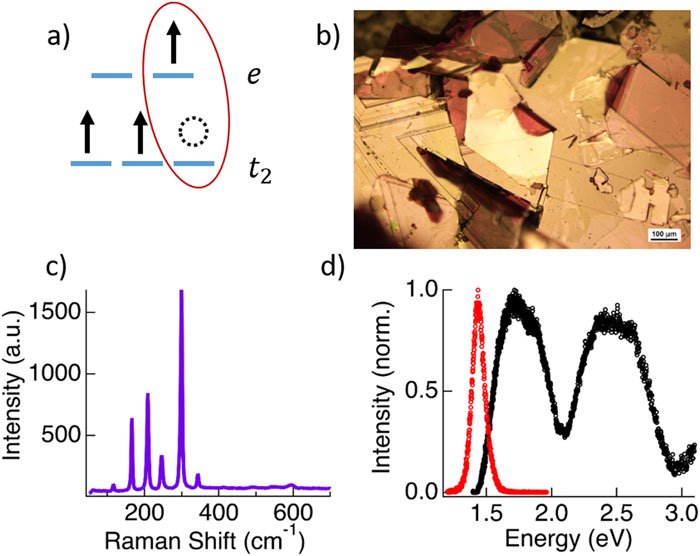
[7] S. Sridhar, S. Khansari, S. O’Donnell, A. T. Barth, E. O. Danilov, F. N. Castellano, P. A. Maggard, D. B. Dougherty.* “Ligand Field Exciton Annihilation in Bulk CrCl₃” J. Chem. Phys. 2024, 161, 114706.
Chromium(III) molecules have persistent emission originating from ligand-field excited states. In collaboration with the Dougherty group (NCSU), we propose a ligand-field assignment for this emission band in solid-state materials.
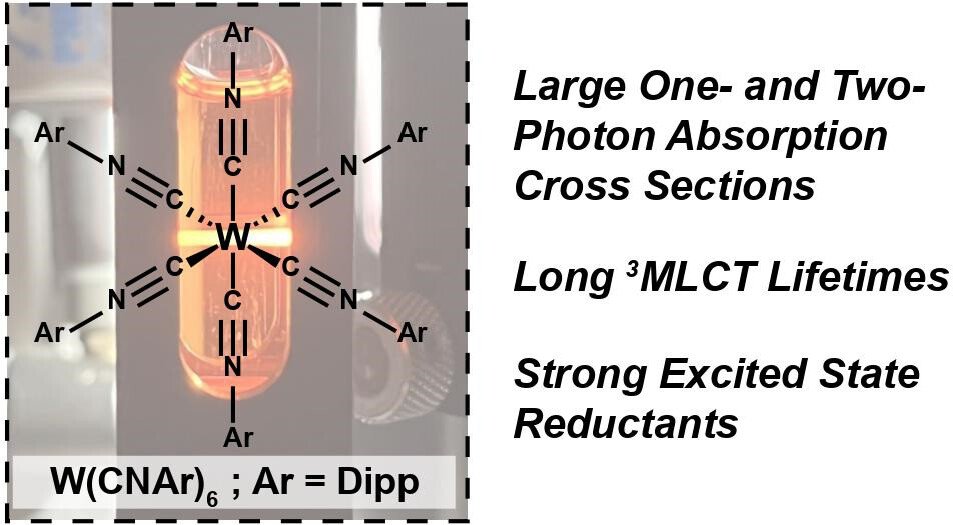
[6] A. T. Barth†, J. Fajardo Jr.†, W. Sattler, J. R. Winkler,* H. B. Gray,* “Electronic Structures and Photoredox Chemistry of Tungsten (0) Arylisocyanides.” Acc. Chem. Res. 2023, 56, 14, 1978–1989.
We establish low-valent tungsten(0) complexes as a new category of earth-abundant 5d photoredox catalysis, reflecting on initial observations on these molecules the Gray lab in the 1970s, and our efforts to repurpose these materials as exceptionally photostable molecules for photoredox catalysis.
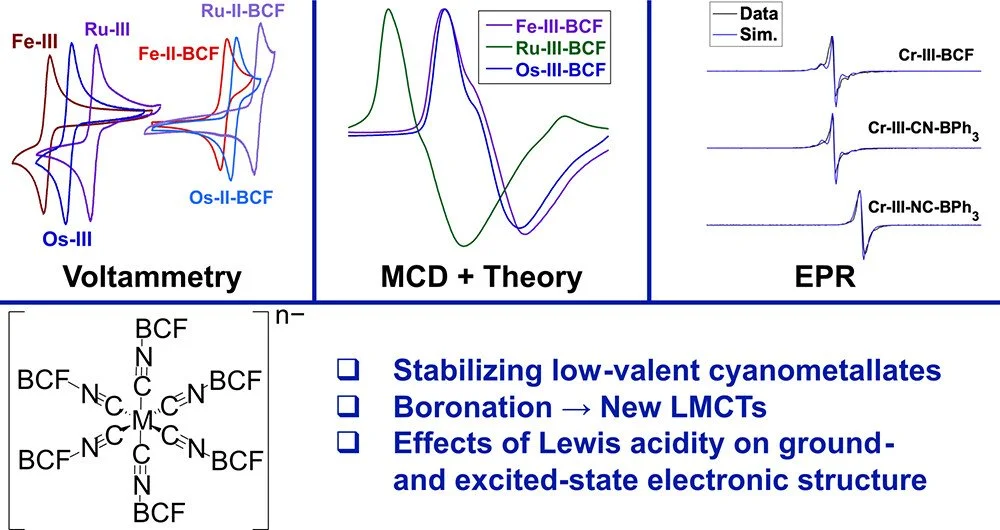
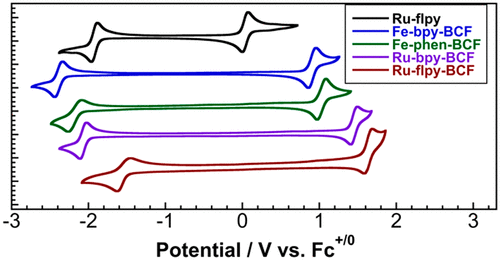
[5] B. J. McNicholas,* C. Nie.; A. Jose, P. H. Oyala, M. K. Takase, L. M. Henling, A. T. Barth, A. Amaolo, R. G. Hadt, E. I. Solomon, J. R. Winkler, H. B. Gray,* E. Despagnet-Ayoub.* “Boronated Cyanometallates” Inorg. Chem. 2023, 62 (7), 2959–2981.
We examine the electronic influence of noncoordinating boranes appended to cyanometallate motifs, which yield large potential shifts and stabilize exotic oxidation states. These species are useful for non-aqueous redox flow batteries.
[4] A. T. Barth, M. Morales, J. R. Winkler,* H. B. Gray,* “Photoredox Catalysis Mediated by Tungsten(0) Arylisocyanides in 1,2-Difluorobenzene” Inorg. Chem. 2022, 61, 19, 7251–7255.
We manipulate solvent polarity to drive photocatalysis with our tungsten(0) arylisocyanide photocatalysts, recognizing that cage-escape yield is a likely bottleneck. This work outlines rate enhancements in the presence of a polar solvent (1,2-difluorobenzene) and with the addition of solvated electrolyte.
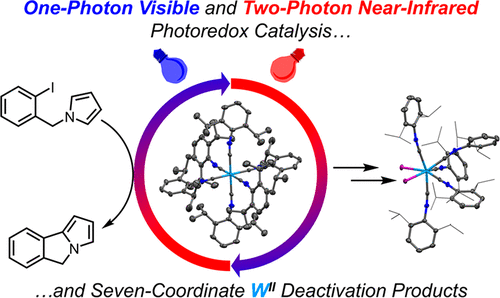
[3] J. Fajardo Jr., A. T. Barth, M. Morales, M. K. Takase, J. R. Winkler,* H. B. Gray,* “Photoredox Catalysis Mediated by Tungsten(0) Arylisocyanides.” J. Am. Chem. Soc. 2021, 143, 46, 19389–19398.
We demonstrate that tungsten(0) photocatalysts can function as potent photoreductant using either UV (400 nm) or two-photon absorption using NIR (800 nm) light. This is a pioneering example of red-light-driven photoredox catalysis applied to reductive dehalogenation reactions for C–C bond formation.
[2] D. X. Ngo, S. A. Del Ciello, A. T. Barth, R. G. Hadt, R. H. Grubbs, H. B. Gray,* B. J. McNicholas,* “Electronic Structures and Electrochemistry of [M(diamine)(CNBR3)4]2- (M = Fe, Ru; R = Ph, C6F5) Complexes.” Inorg. Chem. 2020, 59, 14, 9594–9604.
Boranes act as Lewis acids and can be used to manipulate the electronic structure of cyanometallates. Here, we investigate these influences on di(immine) complexes to examine changes in their photophysical properties.
[1] N. Higdon, A. T. Barth, P. Kozlowski, and R. G. Hadt,* “Spin–Phonon Coupling and Dynamic Zero-Field Splitting Contributions to Spin Conversion Processes in Iron(II) Complexes.” J. Chem. Phys. 2020, 152 (20), 204306.
Molecular motions govern spin-crossover dynamics in pseudo-octahedral Fe(II) complexes. Here, we used CASSCF-NEVPT2 multireference calculations to model changes in the excited-state energy levels along relevant normal modes.